Carbon is known to have more stable compounds than any other element, There are three important properties that allow carbon to form so many stable compounds.
1) Carbon has a fully shared octet of electrons in its compounds.
2) Carbon can form strong single, double and triple bonds to itself.
3) Carbon can form up to 4 covalent bonds.
The diversity of carbon is responsible for the diversity of life, due to carbon being able to form a large variety of compounds means that life can produce a large variety of organisms.
The most common type of bond in Carbon chemistry is the C-H (carbon-hydrogen bond) the study of carbon chemistry is also known as organic chemistry.
Functional groups
The ability of carbon to form strong bonds with carbon and hydrogen leads to the formation of stable compounds, hydrocarbons only contain carbon and hydrogen.
A family of compounds that contain the same functional group is called a homologous series.
some common functional groups are-
Alcohols 

Alkanes 
Ketones 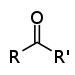

Amines 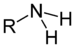
Alkynes 
Ethers 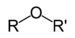
Carboxylic acids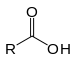

Halogen compounds 
Alkenes 

Aldehydes 

No comments:
Post a Comment